Ideal Gas Laws
Ideal Gas Laws: Overview
This topic covers concepts, such as, Monoatomic, Diatomic and Polyatomic Molecules, Mean Speed of Molecules & Most Probable Speed of Molecules etc.
Important Questions on Ideal Gas Laws
of a gas at a pressure of is compressed to . Taking the temperature to remain constant, the increase in pressure, is
The ratio amongst most probable velocity, mean velocity and root mean square velocity is given by
Under what conditions will a pure sample of an ideal gas not only exhibit a pressure of but also a concentration of ?
Select one correct statement. In the gas equation, PV = nRT
A closed flask contains water in all its three states, solid, liquid and vapour at . In this situation, the average kinetic energy of water molecules will be
If are pressure, volume, molar mass, temperature and gas constant respectively, then for an ideal gas, the density is given by
At what temperature the root-mean-square speed of nitrogen at would be tripled?
From the kinetic theory of gas, prove that where is the density of the gas and is the root mean square velocity of the gas molecules. Show that , where is the absolute temperature.
The saturated water vapour pressure on a planet is, . Determine the vapour density .
A closed vessel contains a mixture of two diatomic gases and . Molar mass of is times that of '' and mass of gas is times that of ''. ThenNumber of molecules of gas in the vessel is eight times that of
In the cylinder shown in the figure, air is enclosed under the piston. Piston mass cross sectional area of the cylinder atmospheric pressure The air temperature is constant, the friction is negligible.
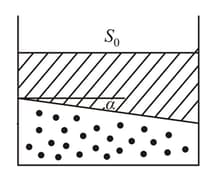
In a cylindrical container of sufficiently large height, two easily moving pistons enclose certain amount of same ideal gas in two chambers as shown in the figure.
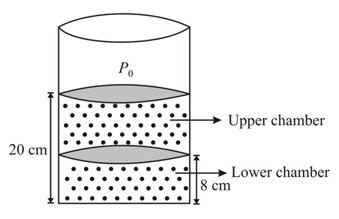
The upper piston is at a height from the bottom and lower piston is at a height from the bottom. The mass of each piston is and cross sectional area of each piston is where and is the atmospheric pressure
The cylindrical container and pistons are made of conducting material. Initially the temperature of gas is and whole system is in equilibrium. Now if the upper piston is slowly lifted by and held in that position with the help of some external force. As a result, the lower piston rises slowly by
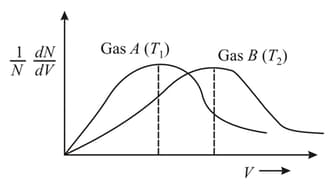
According to Maxwell's distribution of molecular speeds for the below graph for two different samples of ideal gases and at temperature and respectively which of the following statements is/are INCORRECT
The pressure of an ideal gas of moles whose pressure is related to its volume as and are positive constants of appropriate dimensions). The maximum attainable temperature of this ideal gas is when it's volume The value of are
Consider the shown diagram where the two chambers separated by pistonspring arrangement contain equal amounts of certain ideal gas. Initially when the temperatures of the gas in both the chambers are kept at the compression in the spring is The temperature of the left and the right chambers are now raised to and respectively. If the pistons are free to slide, the final compression in the spring (in ) is
On the basis of the kinetic theory of gases if we compare gram of hydrogen with gram of argon. Then,
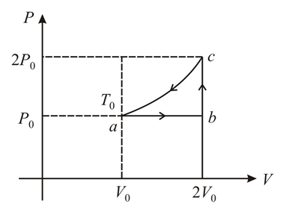
One mole of an ideal monoatomic gas (initial temperature is made to go through the cycle abca shown figure. If denotes the internal energy, then choose the correct statement(s). is a curve)
When an excited atom goes to ground state it emits a photon.
Statement 1 : Mass has changed into energy.
Statement 2 : K.E. has increased.
